Accelerated alkaline hydrogen evolution on M(OH)x/M-MoPOx (M = Ni, Co, Fe, Mn) electrocatalysts by coupling water dissociation and hydrogen ad-desorption steps†
Abstract
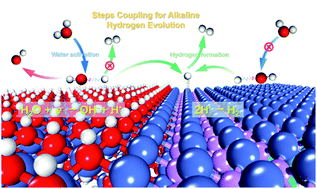
Developing efficient and cheap electrocatalysts for the alkaline hydrogen evolution reaction is still a big challenge due to the sluggish water dissociation kinetics as well as poor M–Had energetics. Herein, hydroxide modification and element incorporation have been demonstrated to realize a synergistic modulation on a new class of M(OH)x/M-MoPOx catalysts for accelerating water dissociation and hydrogen ad-desorption steps in the HER. Theoretical and experimental results disclosed that in situ modification with hydroxide endowed M(OH)x/M-MoPOx with a strong ability to dissociate water, and meanwhile, oxygen incorporation effectively optimized the M–Had energetics of the NiMoP catalyst. Moreover, the interaction between M(OH)x and M-MoPOx components in M(OH)x/M-MoPOx further enhances their ability to catalyze the two elementary steps in alkaline hydrogen evolution, providing a wide avenue for efficiently catalyzing hydrogen evolution. In general, the optimized Ni(OH)2/NiMoPOx catalyst exhibits excellent alkaline HER activity and durability, superior to the state-of-the-art Pt/C catalyst when the overpotential exceeds 65 mV.


 Please wait while we load your content...
Please wait while we load your content...