Copper-catalyzed enantioselective arylalkynylation of alkenes†
Abstract
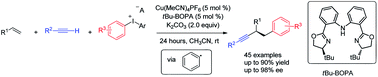
A copper-catalyzed enantioselective arylalkynylation of alkenes with diaryliodonium salt and a monosubstituted alkyne is reported. The three-component coupling reactions proceed under mild reaction conditions with a broad substrate scope, leading to synthetically valuable 1,2-diaryl-3-butynes. The key to the success of this chemistry is the employment of the chiral bisoxazoline-phenylaniline (BOPA) ligand. A novel reaction pathway involving the phenyl radical generation under thermal copper catalysis is proposed according to mechanistic studies.


 Please wait while we load your content...
Please wait while we load your content...