Magnesium-mediated sp3 C–H activation in cascade cyclization of 1-arylethynyl-2-alkyl-o-carboranes: efficient synthesis of carborane-fused cyclopentanes†
Abstract
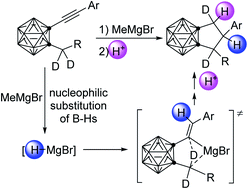
This work reports an unprecedented cascade cyclization of 1-arylethynyl-2-alkyl-o-carboranes promoted by magnesium-mediated sp3 C–H activation. Treatment of 1-arylethynyl-2-alkyl-o-carboranes with MeMgBr gives a series of carborane-fused cyclopentanes in very good yields. Deuterium labelling and control experiments suggest that HMgBr, resulting in situ from the nucleophilic substitution of cage B–H bonds with Grignard reagent, initiates the reaction, in which magnesium-promoted intramolecular sp3 C–H activation serves as a key step. This work not only offers a new route for the synthesis of carborane-fused cyclopentanes, but also sheds some light on Mg-mediated C–H activation and functionalization.


 Please wait while we load your content...
Please wait while we load your content...