A direct route to six and seven membered lactones via γ-C(sp3)–H activation: a simple protocol to build molecular complexity†
Abstract
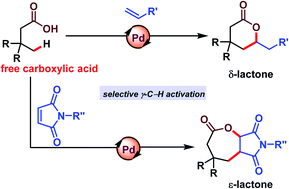
Lactones comprise a class of valuable compounds having biological as well as industrial importance. Development of a methodology to synthesize such molecules directly from readily available materials such as aliphatic carboxylic acid is highly desirable. Herein, we have reported synthesis of δ-lactones and ε-lactones via selective γ-C(sp3)–H activation. The γ-C–H bond containing aliphatic carboxylic acids provide six or seven membered lactones depending on the olefin partner in the presence of a palladium catalyst. A mechanistic investigation suggests that C–H activation is the rate-determining step. Further transformations of the lactones have been carried out to showcase the applicability of the present strategy.
- This article is part of the themed collection: Celebrating the Chemical Sciences in India - Leaders in the Field Symposium 2020


 Please wait while we load your content...
Please wait while we load your content...