Role of ion-selective membranes in the carbon balance for CO2 electroreduction via gas diffusion electrode reactor designs†
Abstract
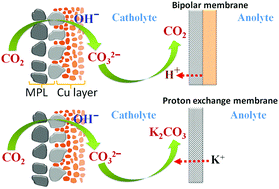
In this work, the effect of ion-selective membranes on the detailed carbon balance was systematically analyzed for high-rate CO2 reduction in GDE-type flow electrolyzers. By using different ion-selective membranes, we show nearly identical catalytic selectivity for CO2 reduction, which is primarily due to a similar local reaction environment created at the cathode/electrolyte interface via the introduction of a catholyte layer. In addition, based on a systematic exploration of gases released from electrolytes and the dynamic change of electrolyte speciation, we demonstrate the explicit discrepancy in carbon balance paths for the captured CO2 at the cathode/catholyte interface via reaction with OH− when using different ion-selective membranes: (i) the captured CO2 could be transported through an anion exchange membrane in the form of CO32−, subsequently releasing CO2 along with O2 in the anolyte, and (ii) with a cation exchange membrane, the captured CO2 would be accumulated in the catholyte in the form of CO32−, while (iii) with the use of a bipolar membrane, the captured CO2 could be released at the catholyte/membrane interface in the form of gaseous CO2. The unique carbon balance path for each type of membrane is linked to ion species transported through the membranes.


 Please wait while we load your content...
Please wait while we load your content...