Multi-targeting of functional cysteines in multiple conserved SARS-CoV-2 domains by clinically safe Zn-ejectors†
Abstract
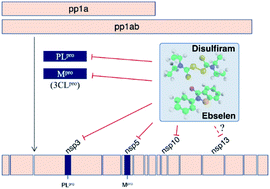
We present a near-term treatment strategy to tackle pandemic outbreaks of coronaviruses with no specific drugs/vaccines by combining evolutionary and physical principles to identify conserved viral domains containing druggable Zn-sites that can be targeted by clinically safe Zn-ejecting compounds. By applying this strategy to SARS-CoV-2 polyprotein-1ab, we predicted multiple labile Zn-sites in papain-like cysteine protease (PLpro), nsp10 transcription factor, and nsp13 helicase. These are attractive drug targets because they are highly conserved among coronaviruses and play vital structural/catalytic roles in viral proteins indispensable for virus replication. We show that five Zn-ejectors can release Zn2+ from PLpro and nsp10, and clinically-safe disulfiram and ebselen can not only covalently bind to the Zn-bound cysteines in both proteins, but also inhibit PLpro protease. We propose combining disulfiram/ebselen with broad-spectrum antivirals/drugs to target different conserved domains acting at various stages of the virus life cycle to synergistically inhibit SARS-CoV-2 replication and reduce the emergence of drug resistance.
- This article is part of the themed collections: Coronavirus articles - free to access collection and Celebrating five years of ChemRxiv


 Please wait while we load your content...
Please wait while we load your content...