Molecular chirality mediated amyloid formation on phospholipid surfaces†
Abstract
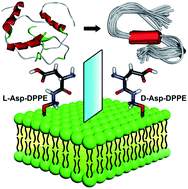
One of the neuropathological features of Alzheimer's disease (AD) is the misfolding of amyloid-β to form amyloid aggregates, a process highly associated with biological membranes. However, how molecular chirality affects the amyloid formation on phospholipid surfaces has seldom been reported. Here, L- and D-aspartic acid-modified 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine (L-/D-Asp–DPPE) is synthesized to construct chiral phospholipid bilayers. We discover that the L-Asp–DPPE liposomes slightly inhibit the Aβ(1–40) nucleation process but cannot affect the oligomer elongation process. By contrast, the D-Asp–DPPE liposomes strongly inhibit both nucleation and elongation of the peptide. Notably, L- and D-Asp–DPPE liposomes not only have good biocompatibility but can also rescue Aβ(1–40)-aggregation induced cytotoxicity with significant chiral discrimination, in which the cell viability is higher in the presence of D-Asp–DPPE liposomes. Mechanism analysis and molecular dynamics simulation clearly demonstrate that differential electrostatic interactions of Lys16 in Aβ(1–40) with L- or D-Asp on the phospholipid contribute to the remarkable chiral discrimination. This study provides a deeper understanding of the crucial amyloidosis process from the perspective of the chiral interface and reveals that the convergence of D-amino acids with the liposomes might be a feasible route for AD prevention.
- This article is part of the themed collections: Amyloids and Protein Aggregation and Chiral Nanomaterials


 Please wait while we load your content...
Please wait while we load your content...