BNN-1,3-dipoles: isolation and intramolecular cycloaddition with unactivated arenes†
Abstract
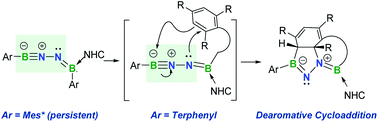
The mono-base-stabilized 1,2-diboranylidenehydrazine derivatives featuring a 1,3-dipolar BNN skeleton are obtained by dehydrobromination of [ArB(Br)NH]2 (Ar = 2,6-diphenylphenyl (Dpp), Ar = 2,6-bis(2,4,6-trimethylphenyl)phenyl (Dmp) or Ar = 2,4,6-tri-tert-butylphenyl (Mes*)) with N-heterocyclic carbenes (NHCs). Depending on the Ar substituents, such species can be isolated as a crystalline solid (Ar = Mes*) or generated as reactive intermediates undergoing spontaneous intramolecular aminoboration of the proximal arene rings via [3 + 2] cycloaddition (Ar = Dpp or Dmp). The latter reactions showcase the 1,3-dipolar reactivity toward unactivated arenes at ambient temperature. In addition, double cycloaddition of the isolable BNN species with two CO2 molecules affords a bicyclic species consisting of two fused five-membered BN2CO rings. The electronic structures of these BNN species and the mechanisms of these cascade reactions are interrogated through density functional theory (DFT) calculations.
- This article is part of the themed collections: Celebrating 100 Years of Chemistry at Nankai University, Spotlighting main group elements in polynuclear complexes and 2020 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...