Palladium-catalysed C–F alumination of fluorobenzenes: mechanistic diversity and origin of selectivity†
Abstract
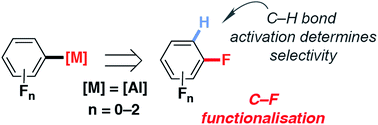
A palladium pre-catalyst, [Pd(PCy3)2] is reported for the efficient and selective C–F alumination of fluorobenzenes with the aluminium(I) reagent [{(ArNCMe)2CH}Al] (1, Ar = 2,6-di-iso-propylphenyl). The catalytic protocol results in the transformation of sp2 C–F bonds to sp2 C–Al bonds and provides a route to reactive organoaluminium complexes (2a–h) from fluorocarbons. The catalyst is highly active. Reactions proceed within 5 minutes at 25 °C (and at appreciable rates at even −50 °C) and the scope includes low-fluorine-content substrates such as fluorobenzene, difluorobenzenes and trifluorobenzenes. The reaction proceeds with complete chemoselectivity (C–F vs. C–H) and high regioselectivities (>90% for C–F bonds adjacent to the most acidic C–H sites). The heterometallic complex [Pd(PCy3)(1)2] was shown to be catalytically competent. Catalytic C–F alumination proceeds with a KIE of 1.1–1.3. DFT calculations have been used to model potential mechanisms for C–F bond activation. These calculations suggest that two competing mechanisms may be in operation. Pathway 1 involves a ligand-assisted oxidative addition to [Pd(1)2] and leads directly to the product. Pathway 2 involves a stepwise C–H → C–F functionalisation mechanism in which the C–H bond is broken and reformed along the reaction coordinate, guiding the catalyst to an adjacent C–F site. This second mechanism explains the experimentally observed regioselectivity. Experimental support for this C–H activation playing a key role in C–F alumination was obtained by employing [{(MesNCMe)2CH}AlH2] (3, Mes = 2,4,6-tri-methylphenyl) as a reagent in place of 1. In this instance, the kinetic C–H alumination intermediate could be isolated. Under catalytic conditions this intermediate converts to the thermodynamic C–F alumination product.


 Please wait while we load your content...
Please wait while we load your content...