Water-induced formation of an alkali-ion dimer in cryptomelane nanorods†
Abstract
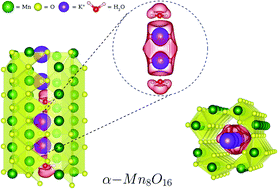
Tunneled metal oxides such as α-Mn8O16 (hollandite) have proven to be compelling candidates for charge-storage materials in high-density batteries. In particular, the tunnels can support one-dimensional chains of K+ ions (which act as structure-stabilizing dopants) and H2O molecules, as these chains are favored by strong H-bonds and electrostatic interactions. In this work, we examine the role of water molecules in enhancing the stability of K+-doped α-Mn8O16 (cryptomelane). The combined experimental and theoretical analyses show that for high enough concentrations of water and tunnel-ions, H2O displaces K+ ions from their natural binding sites. This displacement becomes energetically favorable due to the formation of K2+ dimers, thereby modifying the stoichiometric charge of the system. These findings have potentially significant technological implications for the consideration of cryptomelane as a Li+/Na+ battery electrode. Our work establishes the functional role of water in altering the energetics and structural properties of cryptomelane, an observation that has frequently been overlooked in previous studies.


 Please wait while we load your content...
Please wait while we load your content...