An electrochemically reversible lattice with redox active A-sites of double perovskite oxide nanosheets to reinforce oxygen electrocatalysis†
Abstract
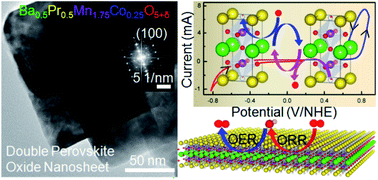
The catalyst surface undergoes reversible structural changes while influencing the rate of redox reactions, the atomistic structural details of which are often overlooked when the key focus is to enhance the catalytic activity and reaction yield. We achieve chemical synthesis of ∼5 unit cell thick double perovskite oxide nanosheets (NSs) and demonstrate their precise structural reversibility while catalyzing the successive oxygen evolution and reduction reactions (OER/ORR). 4.1 nm thick A-site ordered BaPrMn1.75Co0.25O5+δ (δ = 0.06–0.17) NSs with oxygen deficient PrOx terminated layers have flexible oxygen coordination of Pr3+ ions, which promotes the redox processes. When subjected to systematic oxidation and reduction cycles by cyclic voltammetry under small electrochemical bias, the PrO1.8 phase appears and disappears alternately at the NS surface, due to the intake and release of oxygen, respectively. The structural reversibility is attributed to the two-dimensional morphology and the A-site terminated surface with flexible anion stoichiometry. Although the underlying B-site cations are well-known active sites, this is the first demonstration of A(Pr3+)-site cations influencing the activity by reversibly altering their oxygen coordination. Higher Co-doping thwarts the NS formation, affecting the catalytic performance. The facile OER/ORR activity of the thickness-tunable NSs has larger implications as a bifunctional air-electrode material for metal–air batteries and fuel cells.


 Please wait while we load your content...
Please wait while we load your content...