Palladium-catalyzed asymmetric hydrophosphorylation of alkynes: facile access to P-stereogenic phosphinates†‡
Abstract
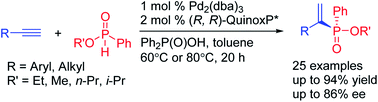
Despite the importance of P-chiral organophosphorus compounds in asymmetric catalysis, transition metal-catalyzed methods for accessing P-chiral phosphine derivatives are still limited. Herein, a catalytic enantioselective method for the synthesis of P-stereogenic alkenylphosphinates is developed through asymmetric hydrophosphorylation of alkynes. This process is demonstrated for a wide range of racemic phosphinates and leads to diverse P-stereogenic alkenylphosphinates directly.


 Please wait while we load your content...
Please wait while we load your content...