Nanomolecular singlet oxygen photosensitizers based on hemiquinonoid-resorcinarenes, the fuchsonarenes†
Abstract
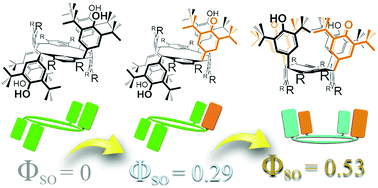
Singlet oxygen sensitization involving a class of hemiquinonoid-substituted resorcinarenes prepared from the corresponding 3,5-di-t-butyl-4-hydroxyphenyl-substituted resorcinarenes is reported. Based on variation in the molecular structures, quantum yields comparable with that of the well-known photosensitizing compound meso-tetraphenylporphyrin were obtained for the octabenzyloxy-substituted double hemiquinonoid resorcinarene reported herein. The following classes of compounds were studied: benzyloxy-substituted resorcinarenes, acetyloxy-substituted resorcinarenes and acetyloxy-substituted pyrogallarenes. Single crystal X-ray crystallographic analyses revealed structural variations in the compounds with conformation (i.e., rctt, rccc, rcct) having some influence on the identity of hemiquinonoid product available. Multiplicity of hemiquinonoid group affects singlet oxygen quantum yield with those doubly substituted being more active than those containing a single hemiquinone. Compounds reported here lacking hemiquinonoid groups are inactive as photosensitizers. The term ‘fuchsonarene’ (fuchson + arene of resorcinarene) is proposed for use to classify the compounds.


 Please wait while we load your content...
Please wait while we load your content...