Catalytic enantioselective synthesis of tetrasubstituted chromanones via palladium-catalyzed asymmetric conjugate arylation using chiral pyridine-dihydroisoquinoline ligands†
Abstract
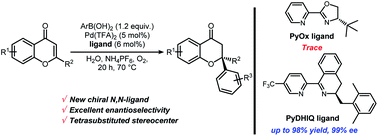
Highly enantioselective conjugate addition reactions of arylboronic acids to 2-substituted chromones catalyzed by palladium complexes with new chiral Pyridine-Dihydroisoquinoline (PyDHIQ) ligands have been developed. These reactions provide highly enantioselective access to chromanones containing tetrasubstituted stereocenters. Various arylboronic acids and 2-substituted chromones can be used in the catalytic reaction to afford the chiral tetrasubstituted chromanones in good yields and excellent enantioselectivities (25 examples, up to 98% yields, up to 99% ee).
- This article is part of the themed collections: Celebrating the 75th Anniversary of the Korean Chemical Society (KCS) and 2020 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...