Unravelling the effect of the E545K mutation on PI3Kα kinase†
Abstract
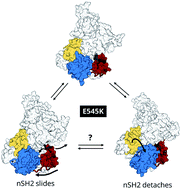
PI3Kα controls several cellular processes and its aberrant signalling is implicated in tumorigenesis. One of its hotspot mutations, E545K, increases PI3Kα lipid kinase activity, but its mode of action is only partially understood. Here, we perform biased and unbiased molecular dynamics simulations of PI3Kα and uncover, for the first time, the free energy landscape of the E545K PI3Kα mutant. We reveal the mechanism by which E545K leads to PI3Kα activation in atomic-level detail, which is considerably more complex than previously thought.


 Please wait while we load your content...
Please wait while we load your content...