Enantioseparation and chiral induction in Ag29 nanoclusters with intrinsic chirality†
Abstract
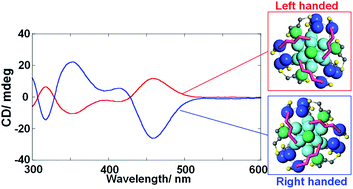
The optical activity of a metal nanocluster (NC) is induced either by an asymmetric arrangement of constituents or by a dissymmetric field of a chiral ligand layer. Herein, we unveil the origin of chirality in Ag29 NCs, which is attributed to the intrinsically chiral atomic arrangement. The X-ray crystal structure of a Ag29(BDT)12(TPP)4 NC (BDT: 1,3-benzenedithiol; TPP: triphenylphosphine) manifested the presence of intrinsic chirality in the outer shell capping the icosahedral achiral Ag13 core. The enantiomers of the Ag29(BDT)12(TPP)4 NC are separated by high-performance liquid chromatography (HPLC) using a chiral column for the first time, showing mirror-image circular dichroism (CD) spectra. The CD spectra are reproduced by time-dependent density functional theory (TDDFT) calculations based on enantiomeric Ag29 models with achiral 1,3-propanedithiolate ligands. The mechanism of chiral induction in the synthesis of Ag29(DHLA)12 (DHLA: α-dihydrolipoic acid) NCs with a chiral ligand system is further discussed with the aid of DFT calculations. The use of the enantiomeric DHLA ligand preferentially leads to a one-handed atomic arrangement which is more stable than the opposite one, inducing the enantiomeric excess in the population of intrinsically chiral Ag29 NCs with CD activity.
- This article is part of the themed collection: Chiral Nanomaterials


 Please wait while we load your content...
Please wait while we load your content...