Enantioselective nickel-catalyzed arylative and alkenylative intramolecular 1,2-allylations of tethered allene–ketones†
Abstract
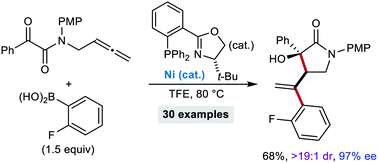
The enantioselective nickel-catalyzed reaction of tethered allene–ketones with (hetero)arylboronic acids or potassium vinyltrifluoroborate is described. Carbonickelation of the allene gives allylnickel species, which undergo cyclization by 1,2-allylation to produce chiral tertiary-alcohol-containing aza- and carbocycles in high diastereo- and enantioselectivities.


 Please wait while we load your content...
Please wait while we load your content...