Singlet fission in a hexacene dimer: energetics dictate dynamics†
Abstract
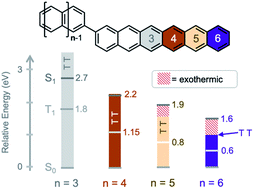
Singlet fission (SF) is an exciton multiplication process with the potential to raise the efficiency limit of single junction solar cells from 33% to up to 45%. Most chromophores generally undergo SF as solid-state crystals. However, when such molecules are covalently coupled, the dimers can be used as model systems to study fundamental photophysical dynamics where a singlet exciton splits into two triplet excitons within individual molecules. Here we report the synthesis and photophysical characterization of singlet fission of a hexacene dimer. Comparing the hexacene dimer to analogous tetracene and pentacene dimers reveals that excess exoergicity slows down singlet fission, similar to what is observed in molecular crystals. Conversely, the lower triplet energy of hexacene results in an increase in the rate of triplet pair recombination, following the energy gap law for radiationless transitions. These results point to design rules for singlet fission chromophores: the energy gap between singlet and triplet pair should be minimal, and the gap between triplet pair and ground state should be large.


 Please wait while we load your content...
Please wait while we load your content...