Brønsted acid catalysis of photosensitized cycloadditions†
Abstract
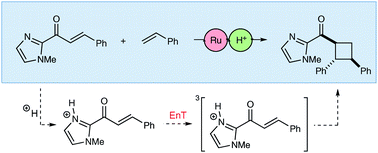
Catalysis is central to contemporary synthetic chemistry. There has been a recent recognition that the rates of photochemical reactions can be profoundly impacted by the use of Lewis acid catalysts and co-catalysts. Herein, we show that Brønsted acids can also modulate the reactivity of excited-state organic reactions. Brønsted acids dramatically increase the rate of Ru(bpy)32+-sensitized [2 + 2] photocycloadditions between C-cinnamoyl imidazoles and a range of electron-rich alkene reaction partners. A combination of experimental and computational studies supports a mechanism in which the Brønsted acid co-catalyst accelerates triplet energy transfer from the excited-state [Ru*(bpy)3]2+ chromophore to the Brønsted acid activated C-cinnamoyl imidazole. Computational evidence further suggests the importance of driving force as well as geometrical reorganization, in which the protonation of the imidazole decreases the reorganization penalty during the energy transfer event.
- This article is part of the themed collection: New reactivity in organic chemistry


 Please wait while we load your content...
Please wait while we load your content...