Palladium mediated domino reaction: synthesis of isochromenes under aqueous medium†
Abstract
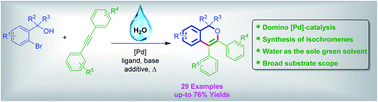
Isochromenes have been synthesized using palladium-catalyzed C–C and C–O bond forming reactions starting from ortho-bromo tertiary benzylic alcohols and internal acetylenes. Notably, this domino process is feasible by using the green solvent, water. The protocol exhibited a broad substrate scope and afforded various isochromenes.


 Please wait while we load your content...
Please wait while we load your content...