Nanocellulose enriches enantiomers in asymmetric aldol reactions†
Abstract
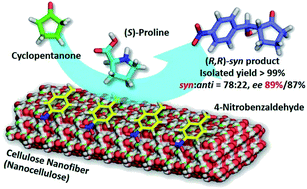
Cellulose nanofibers obtained from wood pulp by TEMPO-mediated oxidation acted as a chiral enhancer in direct aldol reactions of 4-nitrobenzaldehyde and cyclopentanone with (S)-proline as an organocatalyst. Surprisingly, catalytically inactive TEMPO-oxidized cellulose nanofibers enriched the (R,R)-enantiomer in this reaction, affording 89% ee in the syn form with a very high yield (99%). Conversely, nanocellulose-free (S)-proline catalysis resulted in poor selectivity (64% ee, syn form) with a low yield (18%). Green organocatalysis occurring on nanocellulose solid surfaces bearing regularly aligned chiral carbons on hydrophobic crystalline facets will provide new insight into asymmetric synthesis strategies for interfacial catalysis.
- This article is part of the themed collection: 2020 RSC Advances HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...