Catalyst-free regioselective acetylation of primary hydroxy groups in partially protected and unprotected thioglycosides with acetic acid†
Abstract
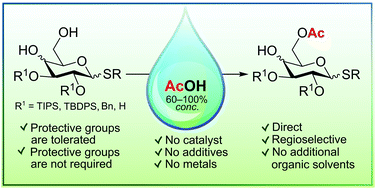
Highly regioselective acetylation of primary hydroxy groups in thioglycoside derivatives with gluco- and galacto-configurations was achieved by treatment with aqueous or anhydrous acetic acid (60–100% AcOH) at elevated temperatures (80–118 °C), avoiding complex, costly and time-consuming manipulations with protective groups. Acetylation of both 4,6-O-benzylidene acetals and the corresponding diols as well as the unprotected tetraol with AcOH was shown to lead selectively to formation of 6-O-acetyl derivatives. For example, the treatment of phenyl 1-thio-β-D-glucopyranoside with anhydrous AcOH at 80 °C for 24 h gave the corresponding 6-O-acetylated derivative in 47% yield (71% based on the reacted starting material) and unreacted starting tetraol in 34% yield, which can easily be recovered by silica gel chromatography and reused in further acetylation.


 Please wait while we load your content...
Please wait while we load your content...