High-temperature resistant water-soluble polymers derived from exotic amino acids†
Abstract
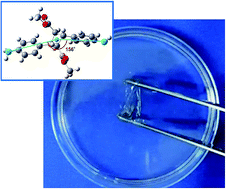
High-performance water-soluble polymers have a wide range of applications from engineering materials to biomedical plastics. However, existing materials are either natural polymers that lack high thermostability or rigid synthetic polymers. Therefore, we design an amino acid-derived building block, 4,4′-diamino-α-truxillate dianion (4ATA2−), that induces water solubility in high-performance polymers. Polyimides containing 4ATA2− units are intrinsically water-soluble and are processed into films cast from an aqueous solution. The resulting polyimide films exhibit exceptional transparency and extremely high thermal stability. In addition, the films can be made insoluble in water by simple post-treatment using weak acid or multivalent metal ions such as calcium. The synthesized polyimide's derived from bio-based resources are useful for yielding waterborne polymeric high-performance applications.


 Please wait while we load your content...
Please wait while we load your content...