Are disulfide bonds resilient to double ionization? Insights from coincidence spectroscopy and ab initio calculations†
Abstract
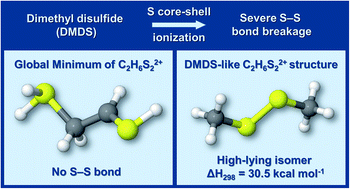
Disulfide bonds (–S–S–) are commonly present in biomolecules and have also been detected in astrophysical environments. In this work, the stability of the disulfide bond towards double ionization is investigated using quantum chemical calculations and photoelectron photoion photoion coincidence (PEPIPICO) spectroscopy measurements on the prototype dimethyl disulfide (CH3SSCH3, DMDS) molecule. The experiments were performed using high energy synchrotron radiation photons before (2465.0 eV) and at (2470.9 eV) the first sigma resonance around the S 1s edge. We applied the multivariate normal distribution analysis to identify the most plausible ionic fragmentation mechanisms from the doubly ionized DMDS. By mapping the minimum energy structures on the dicationic C2H6S22+ potential energy surface, we show that disulfide bonds are only present in high-lying isomers, in contrast to their analogous neutral systems. Our results also indicate that the number of fragment ions containing a disulfide bond for both photon energies is negligible. Taken together, our results reveal that the disulfide bond is severely damaged as a consequence of sulfur core–shell ionization processes, due to the lowering of its thermodynamic stability in multiply-charged systems.


 Please wait while we load your content...
Please wait while we load your content...