Highly fluorescent aryl-cyclopentadienyl ligands and their tetra-nuclear mixed metallic potassium–dysprosium clusters†
Abstract
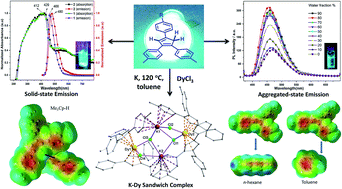
Two alkyl substituted triaryl-cyclopentadienyl ligands [4,4′-(4-phenylcyclopenta-1,3-diene-1,2-diyl)bis(methylbenzene) (1) and 4,4′,4′′-(cyclopenta-1,3-diene-1,2,4-triyl)tris(methylbenzene) (2)] have been synthesized via cross-aldol condensation followed by Zn-dust mediated cyclization and acid catalyzed dehydration reactions. The fluorescence properties of 1 and 2 have been studied in solution and solid state. The ligands exhibited aggregation-induced emission enhancement (AIEE) in THF/water solution. 1 and 2 have been found to be significantly more fluorescent in the solid state than in their respective solutions. This phenomenon can be attributed to the strong intermolecular CH⋯π interactions present in 1 and 2 which leads to the tight packing of molecules in their solid-state. Both 1, 2 and their corresponding anions have been studied by theoretical calculations. Ligands 1 and 2 have been shown to react with anhydrous DyCl3 in the presence of potassium metal at high temperature to afford two fluorescent chloride-bridged tetra-nuclear mixed potassium–dysprosium metallocenes [(Me2Cp)4Dy2IIICl4K2]·3.5(C7H8) (5) and [(Me3Cp)4Dy2IIICl4K2]·3(C7H8) (6), respectively in good yields.
- This article is part of the themed collection: Shining a Light on the f-Block


 Please wait while we load your content...
Please wait while we load your content...