Two-dimensional graphene–HfS2 van der Waals heterostructure as electrode material for alkali-ion batteries†
Abstract
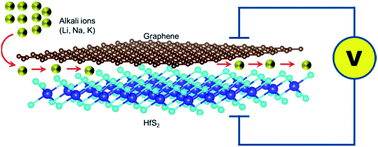
Poor electrical conductivity and large volume expansion during repeated charge and discharge is what has characterized many battery electrode materials in current use. This has led to 2D materials, specifically multi-layered 2D systems, being considered as alternatives. Among these 2D multi-layered systems are the graphene-based van der Waals heterostructures with transition metal di-chalcogenides (TMDCs) as one of the layers. Thus in this study, the graphene–hafnium disulphide (Gr–HfS2) system, has been investigated as a prototype Gr–TMDC system for application as a battery electrode. Density functional theory calculations indicate that Gr–HfS2 van der Waals heterostructure formation is energetically favoured. In order to probe its battery electrode application capability, Li, Na and K intercalants were introduced between the layers of the heterostructure. Li and K were found to be good intercalants as they had low diffusion barriers as well as a positive open circuit voltage. A comparison of bilayer graphene and bilayer HfS2 indicates that Gr–HfS2 is a favourable battery electrode system.


 Please wait while we load your content...
Please wait while we load your content...