Synthesis of isobemisiose, neosartose, and fischerose: three α-1,6-linked trehalose-based oligosaccharides identified from Neosartorya fischeri†
Abstract
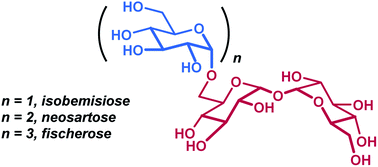
Three complex α-1,6-linked trehalose-based oligosaccharides with unique preservation properties, isobemisiose, neosartose, and fischerose, were recently identified from the extreme stress-tolerant ascospores of Neosartorya fischeri. Herein, we report the first concise, scalable, and iterative chemical synthesis of these oligosaccharides from a differentially protected thioglycoside donor and a selectively protected, asymmetric trehalose acceptor. This work constitutes an improved synthesis of isobemisiose, and is also the first reported synthesis of neosartose, a tetrasaccharide, and fischerose, a pentasaccharide, in good yield. Importantly, in-depth studies of biological function are enabled by this synthetic platform.


 Please wait while we load your content...
Please wait while we load your content...