Natural heterogeneous catalysis with immobilised oxidase biocatalysts†
Abstract
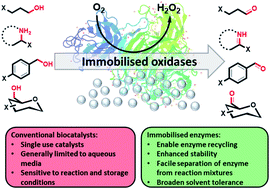
The generation of immobilised oxidase biocatalysts allowing multifunctional oxidation of valuable chemicals using molecular oxygen is described. Engineered galactose oxidase (GOase) variants M1 and M3–5, an engineered choline oxidase (AcCO6) and monoamine oxidase (MAO-N D9) displayed long-term stability and reusability over several weeks when covalently attached on a solid support, outperforming their free counterparts in terms of stability (more than 20 fold), resistance to heat at 60 °C, and tolerance to neat organic solvents such as hexane and toluene. These robust heterogenous oxidation catalysts can be recovered after each reaction and be reused multiple times for the oxidation of different substrates.


 Please wait while we load your content...
Please wait while we load your content...