Synthesis of Schiff and Mannich bases of new s-triazole derivatives and their potential applications for removal of heavy metals from aqueous solution and as antimicrobial agents†
Abstract
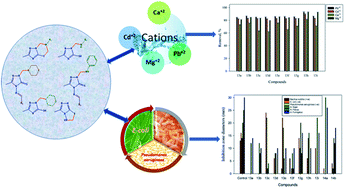
An efficient, simple, and one-pot double Mannich reaction was performed for the synthesis of cyclized 2-methyl-6-substituted-6,7-dihydro-5H-s-triazolo[5,1-b]-1,3,5-thiadiazines via a reaction of 5-methyl-1H-s-triazole-3-thiol (1) with formaldehyde and primary aliphatic amines in ethanol at room temperature, while with primary aromatic amines, uncyclized 3-methyl-1-((substituted-amino)methyl)-1H-s-triazole-5-thiols were produced. Under Mannich reaction conditions, 4-amino-3-methyl-s-triazole-5-thiol (8) reacted with formaldehyde only in boiling ethanol or at room temperature to afford 3-methyl-5,6-dihydro-s-triazolo[3,4-b]-1,3,4-thiadiazole without incorporation of secondary amine. Furthermore, after reaction of compound 8 with aromatic aldehydes under different reaction conditions, uncyclized Schiff's bases were produced. Therefore, reaction of these Schiff's bases with primary or secondary amines with formaldehyde in ethanol at room temperature afforded the corresponding Mannich bases 13–14. The structures of all new compounds were confirmed using spectral analysis. Furthermore, most of the synthesized derivatives showed high efficiency for removal of Pb2+, Cd2+, Ca2+, and Mg2+ from aqueous solutions, as well as antimicrobial activity.


 Please wait while we load your content...
Please wait while we load your content...