Low temperature dehydrogenation properties of ammonia borane within carbon nanotube arrays: a synergistic effect of nanoconfinement and alane†
Abstract
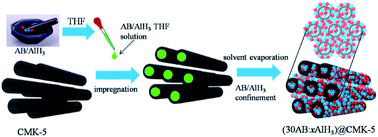
Ammonia borane (AB, NH3BH3) is considered as one of the most promising hydrogen storage materials for proton exchange membrane fuel cells due to its high theoretical hydrogen capacity under moderate temperatures. Unfortunately, its on-board application is hampered by the sluggish kinetics, volatile byproducts and harsh conditions for reversibility. In this work, AB and AlH3 were simultaneously infiltrated into a carbon nanotube array (CMK-5) to combine the synergistic effect of alane with nanoconfinement for improving the dehydrogenation properties of AB. Results showed that the transformation from AB to DADB started at room temperature, which promoted AB to release 9.4 wt% H2 within 10 min at a low temperature of 95 °C. Moreover, the entire suppression of all harmful byproducts was observed.


 Please wait while we load your content...
Please wait while we load your content...