The development of peptide–boron difluoride formazanate conjugates as fluorescence imaging agents†
Abstract
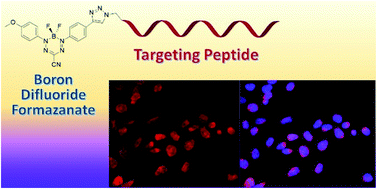
Two new fluorescence imaging probes have been synthesized by incorporating a versatile alkyne-substituted boron difluoride formazanate precursor with peptides through copper-catalyzed alkyne–azide cycloaddition. The formazanate dye was appended to a C-terminal amino acid of ghrelin for imaging the growth hormone secretagogue receptor (GHSR-1a). To demonstrate versatile bioconjugation chemistry, the formazanate dye was added to the N-terminus of bombesin for targeting the gastrin releasing peptide receptor (GRPR). These are the first examples of using this emerging class of dyes, boron difluoride formazanates, for the labelling of biomolecules.


 Please wait while we load your content...
Please wait while we load your content...