Effect of polyethylene glycol on crystal growth and photocatalytic activity of anatase TiO2 single crystals
Abstract
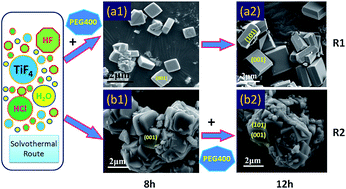
In order to evaluate the effect of polyethylene glycol (PEG) on the growth of TiO2 crystals, anatase TiO2 crystals with different morphologies and structures were synthesized by controlling the content and type of PEG in a solvothermal system. Then, their morphology and structure were characterized by X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), scanning electron microscopy (SEM) and transmission electron microscopy (TEM). Characterization results show that hydrofluoric acid can promote the formation of high activity (001) facets. Experiments on the effect of PEG on crystal growth show that the low molecular weight PEG (PEG400) can accelerate crystal differentiation and relieve the agglomeration of crystals in the presence of hydrofluoric acid. Besides, according to the experimental results, we found that PEG400 can reduce the agglomeration size and number of TiO2 polycrystalline particles. Research on the photocatalytic activity proposed that the independence of single crystal and the integrity of (001) facets are the critical factors in advanced oxidation reaction. The resultant anatase TiO2 single crystals could produce more hydroxyl radicals (˙OH) in the photocatalytic system, which exhibited remarkable photocatalytic performance for the degradation of Acid Red B.


 Please wait while we load your content...
Please wait while we load your content...