Promotion effect of urchin-like MnOx@PrOx hollow core–shell structure catalysts for the low-temperature selective catalytic reduction of NO with NH3
Abstract
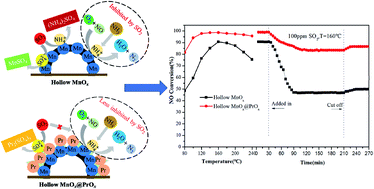
A MnOx@PrOx catalyst with a hollow urchin-like core–shell structure was prepared using a sacrificial templating method and was used for the low-temperature selective catalytic reduction of NO with NH3. The structural properties of the catalyst were characterized by FE-SEM, TEM, XRD, BET, XPS, H2-TPR and NH3-TPD analyses, and the performance of the low-temperature NH3-SCR was also tested. The results show that the catalyst with a molar ratio of Pr/Mn = 0.3 exhibited the highest NO conversion at nearly 99% at 120 °C and NO conversion greater than 90% over the temperature range of 100–240 °C. Also, the MnOx@PrOx catalyst presented desirable SO2 and H2O resistance in 100 ppm SO2 and 10 vol% H2O at the space velocity of 40 000 h−1 and a testing time of 3 h test at 160 °C. The excellent low-temperature catalytic activity of the catalyst could ultimately be attributed to high concentrations of Mn4+ and adsorbed oxygen species on the catalyst surface, suitable Lewis acidic surface properties, and good reducing ability. Additionally, the enhanced SO2 and H2O resistance of the catalyst was primarily ascribed to its unique core–shell structure which prevented the MnOx core from being sulfated.


 Please wait while we load your content...
Please wait while we load your content...