Smart regioselectivity towards mono 6-hydroxyl α-cyclodextrin amphiphilic derivatives†
Abstract
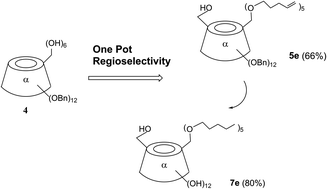
Following the trend of eco-friendly development, a smart regioselective modification is described herein, for mono 6-hydroxyl and penta-alkyl coexistence on the primary face of α-cyclodextrins with no additional catalysis or no enzyme process, just via the adjustment of the ratio of alkali to alkylation agent, with good yields. The novel procedure minimized the tedious protection, deprotection steps and provided useful intermediates for further cutting edge research. Thus, the scope of green and economical access is extended from penta-pentenyl substitution to C4–C6 alkyl group substitution. It was speculated that the mechanism might be controlled by the concentration of alkali in the system and the steric effects of the electrophilic reagent RBr.


 Please wait while we load your content...
Please wait while we load your content...