K2S2O8 mediated C-3 arylation of quinoxalin-2(1H)-ones under metal-, photocatalyst- and light-free conditions†
Abstract
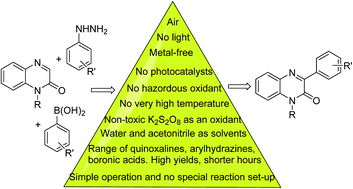
Two facile and effective C-3 arylation protocols of quinoxalin-2(1H)-ones with arylhydrazines and aryl boronic acids respectively via free radical cross-coupling reactions under metal-, photocatalyst- and light-free conditions have been unveiled. K2S2O8 has been used as an efficient oxidant to generate aryl radicals from arylhydrazines and aryl boronic acids under two different reaction conditions. The generated aryl radicals undergo a free radical coupling reaction at the C-3 position of quinoxalin-2(1H)-ones producing 3-arylquinoxalin-2(1H)-ones in good to excellent yields. The involvement of radicals in the course of the reaction has been demonstrated by radical trapping experiments with 2,2,6,6-tetramethylpiperidine-1-oxyl.


 Please wait while we load your content...
Please wait while we load your content...