Three-dimensional NiCoP hollow spheres: an efficient electrode material for hydrogen evolution reaction and supercapacitor applications†
Abstract
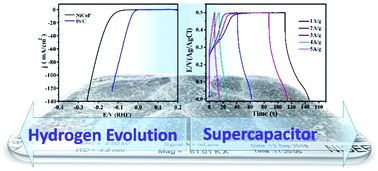
A binary metal phosphide (NiCoP) has been synthesized in a single-step hydrothermal method, and its energy conversion (hydrogen evolution reaction; HER) and energy storage (supercapacitor) performances have been explored. The physicochemical characterization of the NiCoP nanostructures show that they have a highly crystalline phase and are formed uniformly with a sphere-like surface morphology. In acidic electrolytic conditions, the NiCoP shows excellent HER performance, requiring only 160 and 300 mV overpotential to deliver 10 and 300 mA cm−2 current density, respectively. Interestingly, it follows the Volmer–Heyrovsky reaction pathway to execute the HER with robust durability (∼15 mV increase in overpotential even after 18 h of electrolysis). In an alkaline medium (5 M KOH), NiCoP shows specific capacitance of 960 F g−1 with higher energy density (33.3 W h kg−1) and power density (11.8 kW kg−1). Moreover, it shows better reversibility (∼97% coulombic efficiency) and long cycle life (∼95% capacitance retention after 10 000 repeated cycles). The unique surface morphology and phase purity of the binary metal phosphide avails more electroactive surface/redox centers, thereby showing better electrocatalytic as well as energy storage performances. Therefore, we presume that the NiCoP would be a suitable material for future energy conversion and storage systems.


 Please wait while we load your content...
Please wait while we load your content...