Efficient syntheses of macrocycles ranging from 22–28 atoms through spontaneous dimerization to yield bis-hydrazones†
Abstract
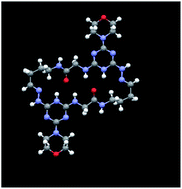
Acid treatment of a triazine displaying both a tethered acetal and BOC-protected hydrazine group leads to spontaneous condensation to yield macrocyclic dimers in excellent yields and purity. The bis-triazinyl hydrazones that form are characterized by 1H-NMR, 13C-NMR, 1H-COSY spectroscopy, X-ray diffraction, and mass spectrometry. By varying the length of the tether—the condensation product of an amino acid and amino acetal—rings comprising 22–28 atoms can be accessed. Glycine and β-alanine were used for the amino acid. The amino acetal comprised 2, 3 or 4 carbon atoms in the backbone. High-performance liquid chromatography (HPLC) was employed to assess purity as well as to fingerprint the six homodimeric products. By combining the protected monomers and subjecting them to acid, mixtures of homodimers and heterodimers are obtained. When all six protected monomers are combined, at least 14 of the 21 theoretical dimeric products are observed by HPLC. Single crystal X-ray diffraction and solution NMR studies reveal the diversity of shapes available to these molecules.


 Please wait while we load your content...
Please wait while we load your content...