Adsorption and anti-corrosion characteristics of vanillin Schiff bases on mild steel in 1 M HCl: experimental and theoretical study†
Abstract
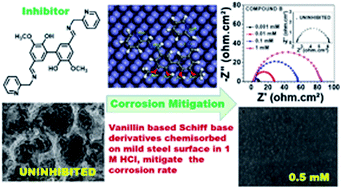
Herein, two Schiff base derivatives of vanillin and divanillin with 2-picolylamine, namely, 2-methoxy-4-((pyridin-2-ylmethylimino)methyl)phenol (compound A) and 3,3′-dimethoxy-5,5′-bis-((pyridin-2-ylmethylimino)methyl)-[1,1′-biphenyl]-2,2′-diol (compound B), respectively, were synthesized. Additionally, their adsorption characteristics and corrosion inhibition behavior were compared for mild steel in 1 M HCl using electrochemical impedance spectroscopy, potentiodynamic polarization and weight loss methods. Compound B was found to impart a better anti-corrosive effect (around 95% inhibition efficiency at 313 K) than compound A. The inhibitors act as effective mixed-type inhibitors and exhibit Langmuir-type adsorption behaviour. The kinetic–thermodynamic parameters together with the data obtained from density functional theory (DFT) and molecular dynamics (MD) simulations illustrate the mechanism of corrosion and mode of adsorption of both inhibitors on the metal surface. The better corrosion mitigation propensity of the dimeric form of the inhibitor (compound B) over the monomeric form (compound A) was tested experimentally and explained according to the theoretical data.


 Please wait while we load your content...
Please wait while we load your content...