Proline derived guanidine catalysts forge extensive H-bonded architectures: a solution and solid state study†
Abstract
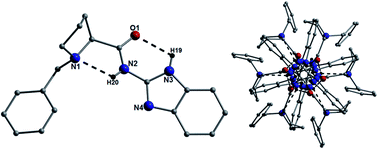
The preparation of a range of amino acid derived guanidine organocatalysts is reported together with their application to the Michael addition of 2-hydroxy-1,4-napthoquinone to β-nitrostyrene, achieving a maximum ee of 56%. Some insight into the mechanism was sought by using X-ray crystallography and a detailed study of the intra- and intermolecular hydrogen bonding is reported.


 Please wait while we load your content...
Please wait while we load your content...