Application of palladium-catalyzed aryl C–H alkylation in total synthesis of (−)-berkelic acid†
Abstract
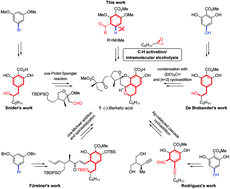
An unprecedented palladium(II)-catalyzed ortho-alkylation of N-methoxybenzamide with epoxides has been applied to the total synthesis of the isochroman natural product (–)-berkelic acid. Combining this strategy with a well documented spiroacetalization cascade reaction, (–)-berkelic acid is obtained in 13.9% overall yield with the longest linear sequence of 11 steps. The results of preliminary biological studies on the synthesized natural product and its analogues are also reported.


 Please wait while we load your content...
Please wait while we load your content...