Ynamides enabled 6-, 7-, and 8-endo-dig iodocyclization of ethoxyethyl ethers: rapid construction of medium-sized oxacycles at room temperature†
Abstract
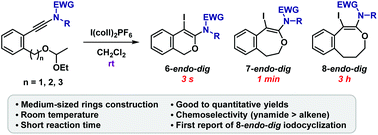
Iodocyclization of ethoxyethyl ethers to ynamides was developed to form six, seven-, and eight-membered cyclic ethers. These reactions reached completion within short reaction times (3 s to 3 h) at room temperature, and the cyclized products were obtained in good to quantitative yields. This is the first report of the construction of medium-sized oxacycles resulting from the iodocyclization of ynamides.


 Please wait while we load your content...
Please wait while we load your content...