Catalytic enantioselective alkylation of 2-alkoxy-tetrahydrofurans†
Abstract
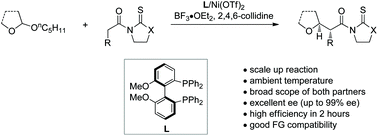
Catalytic asymmetric addition to non-resonance-stabilized oxocarbenium ions has remained a formidable challenge. Herein, we disclosed a nickel(II)-catalyzed asymmetric alkylation of non-resonance-stabilized, five-membered oxocarbenium ions generated in situ from 2-alkoxy tetrahydrofurans with a broad range of carboxylic acid derivatives. The reaction exhibits high efficiency, excellent enantioselectivity, and good functional group tolerance, and can be conducted on a large scale. Aside from five-membered oxocarbenium ions, six-membered and acyclic aliphatic species were also well tolerated with excellent enantiocontrol, thus providing a practical and robust method to access enantiopure α-alkyl substituted saturated ethers.


 Please wait while we load your content...
Please wait while we load your content...