Synthesis, optical and electrochemical properties of propeller-type 3,5,8-trithienyl-BODIPY dyes†
Abstract
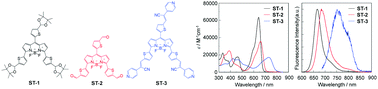
We designed and developed propeller-type 3,5,8-trithienyl-BODIPY dyes ST-1, ST-2, and ST-3, which have the same three units of tetramethyl-2-(thiophen-2-yl)-1,3-dioxolane, thiophene-2-carbaldehyde, or 2-(pyridin-4-yl)-3-(thiophen-2-yl)acrylonitrile at the 3-, 5-, and 8-positions on the BODIPY skeleton, respectively. In order to get an insight into the impacts of 3,5,8-trithienyl substituents on the BODIPY core on the optical and electrochemical properties, we performed the photoabsorption and fluorescence spectroscopy, Lippert–Mataga plots, cyclic voltammetry (CV) and density functional theory (DFT) calculations for ST-1, ST-2, and ST-3. The photoabsorption and fluorescence maxima (λabsmax and λflmax) of the three 3,5,8-trithienyl-DODIPY dyes exhibit bathochromic shifts in the order of ST-1 (ca. 640 and 660 nm) < ST-2 (ca. 660 and 680 nm) < ST-3 (ca. 730 and 750 nm), which appear in significantly longer wavelength regions compared to those of the 3,5,8-triphenyl BODIPY dye. This fact indicates that the expansion of the π-conjugated system by the introduction of thiophene units as spacers at the 3-, 5-, and 8-positions onto the BODIPY core can lead to bathochromic shifts of photoabsorption and fluorescence bands to the red/NIR region. It was found that the photoabsorption and fluorescence bands of the propeller-type 3,5,8-trithienyl-BODIPY dyes are nearly independent of solvent polarity, that is, ST-1, ST-2, and ST-3 show feeble solvatochromic properties. Moreover, we revealed that ST-3 possesses the ability to generate singlet oxygen (1O2) under visible light irradiation, and thus, this result provides useful knowledge in molecular design of efficient BODIPY-based photosensitizers for photodynamic therapy (PDT).


 Please wait while we load your content...
Please wait while we load your content...