Polymers from sugars and unsaturated fatty acids: ADMET polymerisation of monomers derived from d-xylose, d-mannose and castor oil†
Abstract
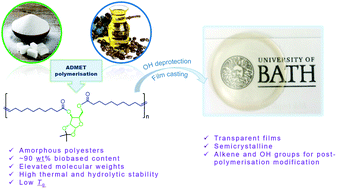
α,ω-Unsaturated glycolipids derived from natural monosaccharides D-xylose and D-mannose, and from a castor oil derivative, 10-undecenoic acid, were synthesised and polymerised via acyclic diene metathesis (ADMET), using Grubbs second generation catalyst. The synthesis of these polymers, which combine a rigid isopropylidene-functionalised carbohydrate core with flexible unsaturated aliphatic chains, was confirmed by NMR spectroscopy and size exclusion chromatography (SEC). The effect of different parameters on the polymerisations were investigated, including temperature, catalyst loading, absence/presence of solvents, effect of a molecular weight moderator and mixing technique. Polyesters with high molecular weights (up to 71 kg mol−1) could be obtained in elevated yields (85%). These amorphous polymers were highly thermally (up to 355 °C) and hydrolytically (pH 7, 0, 14) stable, and showed relatively low glass transition temperatures (−28 to −8 °C), imparted by the flexible fatty acid chain. Deprotection of ketal groups on the polymer backbone was possible up to 72% and changed the material properties, leading to partial crystallinity and insolubility. These partially deprotected polymers allowed the production of transparent thin polymer films and were amenable to further functionalisation.


 Please wait while we load your content...
Please wait while we load your content...