Processable epoxy-telechelic polyalkenamers and polyolefins for photocurable elastomers†
Abstract
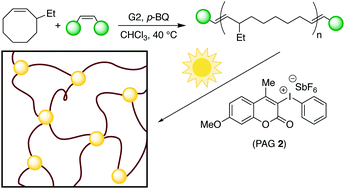
A series of epoxy-telechelic polyalkenamers with varying molar masses were synthesized by ring-opening metathesis polymerization of 3-ethylcyclooctene using a diepoxy-functionalized symmetric chain transfer agent with p-benzoquinone as an additive. An epoxy-telechelic polyolefin was also synthesized using 3-ethylcyclooctene and a carboxybenzyl-protected diol chain transfer agent by ring-opening metathesis polymerization followed by post-polymerization modification. The prepolymers were crosslinked with UV light in the presence of photoacid generators, and network formation was monitored by photorheology. The stiffness and ultimate elongation of the resulting elastomers was tuned by varying molar mass and backbone unsaturation of the prepolymer.


 Please wait while we load your content...
Please wait while we load your content...