Iron-mediated deuterium addition cascade cyano insertion/cyclization of N-arylacrylamides to access deuterium-labelled phenanthridines†
Abstract
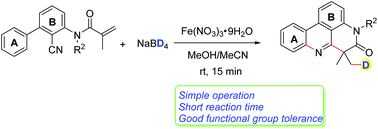
Deuterated molecules feature important biological activities and pharmacokinetic properties for the design of pharmaceuticals. Radical cyclization cascades involving the deuteration of alkenes to form heterocyclic compounds have rarely been reported. Herein, a deuterium addition cascade cyano insertion/cyclization of N-arylacrylamides to synthesize deuterated phenanthridines is described. Cheap Fe(NO3)3·9H2O enables the generation of a deuterium radical from NaBD4in situ. These reactions proceed under mild conditions in a short time and a variety of functional groups are well tolerated.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...