Dearomatization of 3-cyanoindoles by (3 + 2) cycloaddition: from batch to flow chemistry†
Abstract
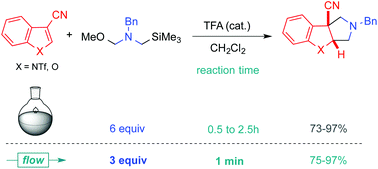
1,3-Dipolar dearomatizing cycloadditions between a non-stabilized azomethine ylide and 3-cyanoindoles or benzofuran afford the corresponding 3D-heterocycles bearing a quaternary carbon centre at the ring junction. While 6 equivalents of ylide precursor 1 are required for full conversion in a classical flask, working under flow conditions limits the excess (3 equiv., tR = 1 min) and leads to a cleaner process, affording cycloadducts that are easier to isolate.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...