Biochemical characterisation of an α1,4 galactosyltransferase from Neisseria weaveri for the synthesis of α1,4-linked galactosides†
Abstract
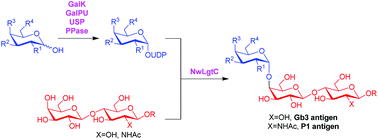
The human cell surface trisaccharide motifs globotriose and P1 antigen play key roles in infections by pathogenic bacteria, which makes them important synthetic targets as antibacterial agents. Enzymatic strategies to install the terminal α1,4-galactosidic linkage are very attractive but have only been demonstrated for a limited set of analogues. Herein, a new bacterial α1,4 galactosyltransferase from N. weaveri was cloned and produced recombinantly in E. coli BL21 (DE3) cells, followed by investigation of its substrate specificity. We demonstrate that the enzyme can tolerate galactosamine (GalN) and also 6-deoxygalactose and 6-deoxy-6-fluorogalactose as donors, and lactose and N-acetyllactosamine as acceptors, leading directly to analogues of Gb3 and P1 that are valuable chemical probes and showcase how biocatalysis can provide fast access to a number of unnatural carbohydrate analogues.
- This article is part of the themed collections: Chemical Biology in OBC, Glycosylation: New methodologies and applications and Celebrating our 2020 Prize and Award winners


 Please wait while we load your content...
Please wait while we load your content...