Selective fluorescence sensing of H2PO4− by the anion induced formation of self-assembled supramolecular polymers†
Abstract
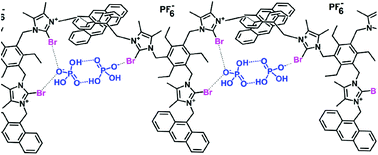
The utilization of anions to induce the formation of self-assembled supramolecular polymers in solution is an undeveloped area of host–guest chemistry. We report in this manuscript a comparative study of two tripodal anion receptors by hydrogen or halogen bonding interactions to form self-assembled supramolecular structures induced by the presence of anions. DOSY NMR and DLS experiments provided evidence for the formation of supramolecular structures in solution in both halogen and hydrogen bond donors with H2PO4− anions. The nucleation and elongation constants obtained using the thermodynamic model indicate that the polymers grow following an isodesmic mechanism. Emission studies demonstrate that only the formation of the supramolecular polymer between the halogen bond donor receptor and H2PO4− anions results in the appearance of the excimer emission band.
- This article is part of the themed collection: Supramolecular chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...