Reagent controlled stereoselective synthesis of teichoic acid α-(1,2)-glucans†
Abstract
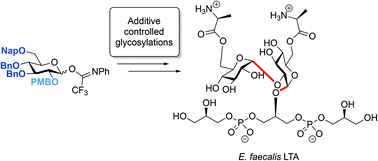
The stereoselective construction of 1,2-cis-glycosidic linkages is key in the assembly of biologically relevant glycans, but remains a synthetic challenge. Reagent-controlled glycosylation methodologies, in which external nucleophiles are employed to modulate the reactivity of the glycosylation system, have become powerful means for the construction of 1,2-cis-glycosidic linkages. Here we establish that nucleophilic additives can support the construction of α-1,2-glucans, and apply our findings in the construction of a D-alanine kojibiose functionalized glycerol phosphate teichoic acid fragment. This latter molecule can be found in the cell wall of the opportunistic Gram-positive bacterium, Enterococcus faecalis and represents a structural element that can possibly be used in the development of therapeutic vaccines and diagnostic tools.
- This article is part of the themed collections: Glycosylation: New methodologies and applications and Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...